
BrightComSol
Scintillator-based technology for radiotherapy dosimetry and imaging.

FLASH Radiotherapy
Status of Current Radiotherapy: With over 19 million new cancer cases and 10 million deaths annually, cancer remains the second leading cause of mortality worldwide. It continues to be one of the most critical global health policy priorities. Despite significant progress, nearly one-third of patients with solid tumors still exhibit resistance to all available treatment modalities. Alongside surgery and systemic therapies such as chemotherapy and immunotherapy, radiotherapy represents a cornerstone of cancer management and is widely recognized as one of the most cost-effective treatment options. Based on current clinical indications, more than half of all cancer patients undergo at least one course of radiotherapy during their disease trajectory, either as a standalone treatment or in combination with other therapeutic approaches.
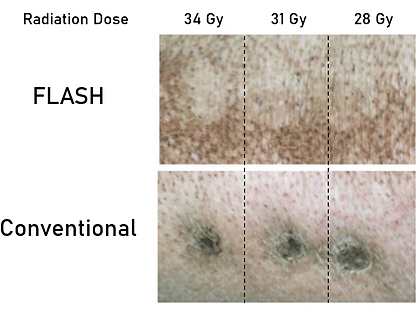
What is FLASH Radiotherapy? FLASH radiotherapy (FLASH-RT) is an emerging paradigm in radiation oncology that delivers ionizing radiation at ultra-high dose rates (≥40 Gy/s), which are more than hundreds of orders of magnitude higher than those used in conventional clinical radiotherapy (0.01–0.1 Gy/s). Despite the substantial difference in dose rate, the total absorbed dose administered to the target tissue remains comparable to that of standard treatments. However, in FLASH-RT, the prescribed dose is deposited within milliseconds rather than over several minutes, as is the case with conventional irradiation protocols. Remarkably, a growing body of preclinical and clinical evidence demonstrates that such ultra-rapid dose delivery can induce equivalent tumoricidal efficacy while simultaneously sparing normal tissues from radiation-induced toxicity. This differential biological response between tumor and healthy tissues, termed the "FLASH effect," represents a potentially transformative advancement in radiotherapy.
FLASH Clinical Trials: Over the past decade, FLASH radiotherapy (FLASH-RT) has evolved from an experimental concept into a rapidly progressing translational research field. After extensive preclinical validation across multiple animal models — demonstrating tumor control comparable to conventional radiotherapy and reduced normal tissue toxicity — the approach is now entering early-stage clinical evaluation.
The first human FLASH treatment was reported in 2019 at the Centre Hospitalier Universitaire Vaudois (CHUV) in Lausanne, Switzerland. In this pioneering case, a single patient with cutaneous T-cell lymphoma received electron-based FLASH radiotherapy, marking the first clinical translation of the FLASH effect in humans. The treatment was delivered using a modified clinical linear accelerator operating at ultra-high dose rates (>40 Gy/s). The results demonstrated complete local tumor control with minimal acute toxicity, supporting the safety and feasibility of FLASH delivery in a clinical setting (Bourhis et al., 2019, Radiotherapy and Oncology).
Building on these findings, subsequent research efforts have focused on proton-based FLASH radiotherapy, which enables deeper dose deposition suitable for treating internal tumors. The FAST-01 (Feasibility of FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases) trial, sponsored by Varian and conducted at the University of Cincinnati, represents the first-in-human clinical study using proton FLASH. Initiated in 2020, this Phase I trial investigates the safety, feasibility, and preliminary efficacy of FLASH proton therapy for patients with painful bone metastases. The study aims to establish the clinical and dosimetric parameters necessary for large-scale implementation of proton FLASH therapy (Daugherty et al., 2023, International Journal of Radiation Oncology, Biology, Physics).
Future studies, such as the FAST-02, LANCE, and ChiCTR2400080935 trials, aim to further validate the reproducibility of the FLASH effect across different tumor types, dose rates, and delivery modalities — ultimately paving the way toward routine clinical adoption.
Dosimetry Challenges in FLASH-RT
The extreme dose rates and short treatment times of FLASH radiotherapy introduce significant challenges for accurate dose measurement and quality assurance. At ultra-high dose rates exceeding 40 Gy/s, traditional dosimeters often saturate or display dose-rate-dependent responses, resulting in inaccurate readings. Moreover, the sub-second treatment durations demand dosimetry systems with exceptionally high temporal resolution to capture the entire dose delivery profile. Another major concern is the high dose per pulse, often greater than 1 Gy, which surpasses the linear response range of many conventional dosimeters and leads to measurement errors. Due to the rapid delivery, real-time monitoring of dose is essential, as conventional sequential measurements are not feasible. Conventional dosimetry tools each face limitations under these conditions. Ionization chambers, for instance, experience ion recombination at high dose rates, causing dose underestimation by up to 30%. Film dosimetry requires post-processing, preventing real-time feedback during treatment. Semiconductor detectors show dose-rate dependence and can suffer from radiation damage at FLASH dose rates. Similarly, electronic portal imaging device (EPID) systems lack the necessary dynamic range and frame rate to monitor such ultra-fast dose deliveries effectively. Collectively, these limitations underscore the urgent need for the development of specialized dosimetry systems tailored for FLASH-RT applications.
Our Solution:
To overcome the critical challenges of FLASH dosimetry, we introduce a revolutionary 2D scintillator dosimetry system that combines ultra-fast scintillation physics with advanced high-speed imaging technology. The core of this system lies in the use of scintillators, which exhibit an ultra-fast response time of less than 1 microsecond and maintain a linear dose response even at ultra-high dose rates. Scintillators convert radiation energy directly into visible light through fluorescence, enabling real-time, saturation-free, and lag-free detection of radiation doses. By coupling high-quality scintillating screens with state-of-the-art camera systems, the setup captures the 2D dose distribution in real-time during FLASH delivery. Advanced imaging and processing algorithms allow instantaneous dose calculation and verification with sub-millisecond temporal resolution. The system enables real-time feedback during treatment delivery, offers immediate quality assurance verification, and exhibits no radiation-induced degradation or signal loss.


ABOUT US
At BrightComSol, our mission is to transform global access to safe and effective radiotherapy. This is possible by unlocking the potential of FLASH radiotherapy, a revolutionary treatment approach where cancer patients receive their entire radiation dose in less than one second, instead of multiple sessions over several weeks. Major radiotherapy machine producers are betting on FLASH technology, but how do they know their multi-million dollar radiation machine is actually delivering the exact prescribed dose during the treatment? Our product, BrightEye™, is an imaging-based real-time dosimetry solution designed to solve this problem by monitoring the radiation beam during the treatment to ensure the radiotherapy machine delivers the exact radiation dose to the cancer patient to avoid over- or under-dose delivery. At BrightComSol, we are making FLASH radiotherapy a clinical reality by working closely with radiotherapy machine producers in a clinical environment.
BrightComSol GmbH is a privately owned nanotechnology spin-off from the BOKU University based in Vienna, Austria. It was founded in March 2020 by Dr. Behzad Shirmardi and Prof. Erik Reimhult. With over a decade of experience in colloidal science and nanomaterial synthesis, they launched the company following the successful development of several key technologies during Dr. Shirmardi’s postdoctoral research. The proprietary technology was invented as part of our fundamental research into core-shell nanoparticles funded by the EU-H2020-ERC-Consolidator Project Interaction and actuation of lipid membranes with magnetic nanoparticles. (MemNP) and EU-H2020-ERC-PoC Project Nanocomposite Solutions (NanoComSol). Since its inception, BrightComSol has been expanding its intellectual property portfolio, now holding more than eight active patents. BrightComsol is supported by Austrian public grants and business angels at 4u-ventures and Mpoweru.














